Metanib 1 Pcs
Alternative products
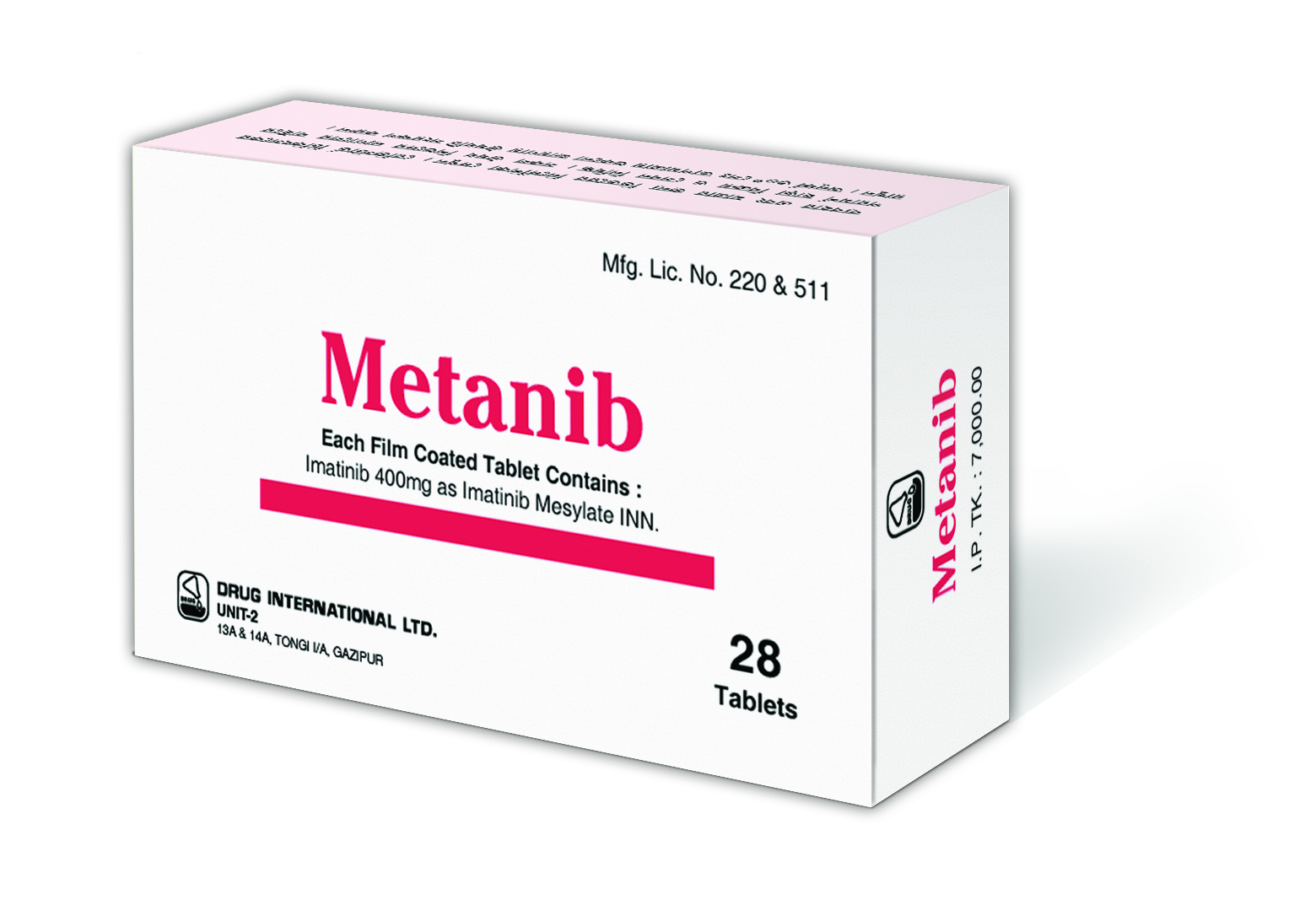
Imatinib Mesylate
Indications
- Newly diagnosed adult and pediatric patients with Philadelphia chromosome positive chronic myeloid leukemia (Ph+ CML) in chronic phase.
- Patients with Philadelphia chromosome positive chronic myeloid leukemia in blast crisis, accelerated phase, or in chronic phase after failure of interferon-alpha therapy.
- Adult patients with relapsed or refractory Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL).
- Pediatric patients with newly diagnosed Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL) in combination with chemotherapy.
- Adult patients with myelodysplastic or myeloproliferative diseases associated with PDGFR (platelet-derived growth factor receptor) gene re arrangements as determined with an FDA-approved test
- Adult patients with aggressive systemic mastocytosis without the D816V c-Kit mutation as determined with an FDA approved test
- Adult patients with hypereosinophilic syndrome and/or chronic eosinophilic leukemia who have the FIP1L1-PDGFRα fusion kinase (mutational analysis or FISHdemonstration of CHIC2 allele deletion) and for patients with HES and/or CEL who are FIP1L1-PDGFRα fusion kinase negative or unknown.
- Adult patients with unresectable, recurrent and/or metastatic dermatofibrosarcoma protuberans.
- Patients with Kit (CD117) positive unresectable and/or metastatic malignant gastrointestinal stromal tumors.
- Adjuvant treatment of adult patients following complete gross resection of Kit (CD117) positive GIST.
Pharmacology
Imatinib, is a tyrosine kinase inhibitor that inhibits the BCR-ABL tyrosine kinase created by the Philadelphia chromosome abnormality in chronic myeloid leukaemia (CML). It blocks proliferation and induces apoptosis in BCR-ABL positive cell lines, as well as fresh leukaemic cells from Philadelphia chromosome positive CML. Imatinib also inhibits receptor kinases for platelet-derived growth factor (PDGF) and stem cell factor (SCF), c-kit, PDGF- and SCF-mediated cellular events.
Dosage
Oral (Adult)-
- Dermatofibrosarcoma protuberans: 400 mg bid.
- Chronic myeloid leukaemia: Chronic phase: 400 mg daily, increased to 600 mg daily or 400 mg bid. Blast crisis or accelerated phase: 600 mg daily, increased to 400 mg bid as required.
- Mastocytosis: 400 mg daily. Start with 100 mg daily if there is associated eosinophilia, may increase to 400 mg if response is insufficient.
- Unresectable, metastatic malignant gastrointestinal stromal tumours: 400 mg daily, may increase up to 400 mg bid.
- Myelodysplastic disease: 400 mg daily.
- Acute lymphoblastic leukaemia, Monotherapy in relapsed or refractory acute lymphoblastic leukaemia: 600 mg daily with induction, consolidation or maintenance chemotherapy.
- Hypereosinophilic syndrome: 400 mg daily. Start with 100 mg daily in patients with FIP1L1-PDGF receptor-α fusion kinase, may increase to 400 mg if response is inadequate.
Oral (Child)-
- Chronic myeloid leukaemia: 340 mg/m2 daily. Max: 800 mg. May be given once daily or divided into morning and evening doses. May be increased to 570 mg/m2 daily in childn w/ disease progression, unsatisfactory haematological response at least 3 mth of treatment, failed to achieve cytogenic response after 12 mth of treatment or loss a previously achieved haematological or cytogenic response.
- Acute lymphoblastic leukaemia, Monotherapy in relapsed or refractory acute lymphoblastic leukaemia: 340 mg/m2 daily. Max: 600 mg.
* চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন'
Administration
Should be taken with food.
* চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন'
Interaction
Increased serum levels with CYP3A4 inhibitors (e.g. azole antifungals, macrolide antibiotics). Reduced serum levels with CYP3A4 inducers (e.g. carbamazepine, dexamethasone, phenobarbital, phenytoin, rifampicin). May increase serum levels of substrates of CYP3A4 (e.g. ciclosporin, pimozide, triazolo-benzodiazepines, dihydropyridine Ca channel blockers, certain statins), CYP2C9 (e.g. warfarin) and CYP2D6.
Contraindications
Hypersensitivity. Lactation.
Side Effects
GI disturbances and bleeding, myelosuppression, superficial oedema, myalgia, arthralgia, muscle cramps, fatigue, rhabdomyolysis, rashes, pruritus, headache, dizziness, taste disturbances, anorexia, paraesthesia, insomnia, eye disorders or visual disturbances, epistaxis, cough, dyspnoea, dry skin, alopecia, night sweats, pyrexia, weakness, rigors, haemorrhages, growth retardation in childn, CHF, palpitations, tachycardia, arrhythmias, angina pectoris and MI. Rarely, serious and severe skin disorders (e.g. erythema multiforme, Stevens-Johnson syndrome, acute generalised exanthematous pustulosis, exfoliative dermatitis and bullous eruptions); aseptic necrosis of bone, ulceration.
Pregnancy & Lactation
Category D: There is positive evidence of human fetal risk, but the benefits from use in pregnant women may be acceptable despite the risk (e.g., if the drug is needed in a life-threatening situation or for a serious disease for which safer drugs cannot be used or are ineffective).
Precautions & Warnings
Patient with cardiac disease or increased risk for CHF. Renal and hepatic impairment. Pregnancy.
Use in Special Populations
Patients on potent CYP3A4 inducers: Increase dose by 50% and carefully monitor clinical response.
Severe Hepatic Impairment: Reduce dose by 25%.
Overdose Effects
Symptoms: Nausea, vomiting, diarrhoea, rash, erythema, oedema, swelling, fatigue, muscle spasms, thrombocytopenia, pancytopenia, headache, pyrexia, decreased appetite, weakness, myalgia, GI pain, decreased neutrophil count, increased creatine phosphokinase, bilirubin and transaminases.
Management: Supportive and symptomatic treatment.
Therapeutic Class
Targeted Cancer Therapy
Storage Conditions
Store below 30° C. Protect from moisture.
- Type Tablet
- Tag
- Morbi leo risus
- Porta ac consectetur ac
- Vestibulum at eros