FXR Tablet 10 mg 1 Pcs
Alternative products
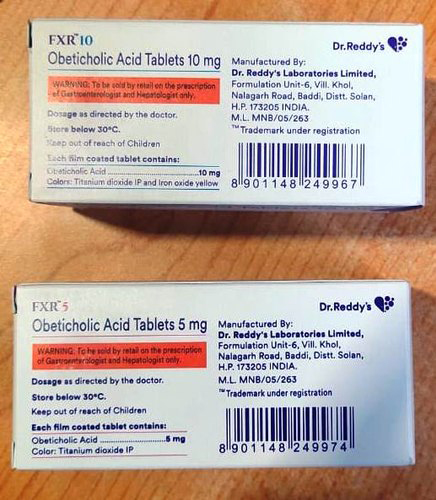
Obeticholic Acid
Indications
Obeticholic Acid is indicated for the treatment of primary biliary cholangitis (PBC) in combination with Ursodeoxycholic Acid (UDCA) in adults with an inadequate response to UDCA, or as monotherapy in adults unable to tolerate UDCA, cholestatic liver disease & non-alcoholic fatty liver disease (NAFLD) including non-alcoholic steatohepatitis (NASH).
Pharmacology
Obeticholic Acid is an agonist for FXR, a nuclear receptor expressed in the liver and intestine. FXR is a key regulator of bile acid, inflammatory, fibrotic, and metabolic pathways. FXR activation decreases the intracellular hepatocyte concentrations of bile acids by suppressing de novo synthesis from cholesterol as well as by increased transport of bile acids out of the hepatocytes. These mechanisms limit the overall size of the circulating bile acid pool while promoting choleresis, thus reducing hepatic exposure to bile acids.
Dosage & Administration
Starting Dosage: The recommended starting dosage of Obeticholic Acid is 5 mg orally once daily in adult patients who have not achieved an adequate biochemical response to an appropriate dosage of Ursodeoxycholic Acid (UDCA) for at least 1 year or are intolerant to UDCA.
Dosage Titration: If an adequate reduction in alkaline phosphatase (ALP) and/or total bilirubin has not been achieved after 3 months of Obeticholic Acid 5 mg once daily, and the patient is tolerating Obeticholic Acid, increase the dosage of Obeticholic Acid to 10 mg once daily.
Maximum Dosage: The maximum recommended dosage of Obeticholic Acid is 10 mg once daily.
Management of Patients with Intolerable Pruritus on Obeticholic Acid: For patients with intolerable pruritus on Obeticholic Acid, consider one or more of the following:
Add an antihistamine or bile acid binding resin. Reduce the dosage of Obeticholic Acid to:
- 5 mg every other day, for patients intolerant to 5 mg once daily
- 5 mg once daily, for patients intolerant to 10 mg once daily
Temporarily interrupt Obeticholic Acid dosing for up to 2 weeks followed by restarting at a reduced dosage
Increase the dosage of Obeticholic Acid to 10 mg once daily, as tolerated, to achieve optimal response. Consider discontinuing Obeticholic Acid treatment in patients who continue to experience persistent, intolerable pruritus.
* চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন'
Interaction
Bile Acid Binding Resins: Bile acid binding resins such as cholestyramine, colestipol, or colesevelam adsorb and reduce bile acid absorption and may reduce the absorption, systemic exposure, and efficacy of Obeticholic Acid. If taking a bile acid binding resin, take Obeticholic Acid at least 4 hours before or 4 hours after taking the bile acid binding resin, or at as great an interval as possible.
Warfarin: The International Normalized Ratio (INR) decreased following co-administration of warfarin and Obeticholic Acid. Monitor INR and adjust the dosage of warfarin, as needed, to maintain the target INR range when co-administering Obeticholic Acid and warfarin.
CYP1A2 Substrates with Narrow Therapeutic Index: Obeticholic Acid may increase the exposure to concomitant drugs that are CYP1A2 substrates. Therapeutic monitoring of CYP1A2 substrates with a narrow therapeutic index.
Contraindications
Contraindicated in patients known to have hypersensitivity to the drug or any of its components & in patients with complete biliary obstruction.
Side Effects
The most common side effects of Obeticholic Acid include: Pruritus, Fatigue & Stomach pain and discomfort. Other common side effects include rash, arthralgia (joint pain), oropharyngeal pain (pain in the middle part of the throat), dizziness, constipation, abnormal thyroid function, and eczema (inflammation of the skin).
Pregnancy & Lactation
The limited available human data on the use of Obeticholic Acid during pregnancy are not sufficient to inform a drug-associated risk.There is no information on the presence of Obeticholic Acid in human milk, the effects on the breast-fed infant or the effects on milk production.
Precautions & Warnings
The following clinically significant reactions are described:
- Liver-Related Adverse Reactions
- Severe Pruritus
- Reduction in high density lipoprotein-cholesterol (HDL-C)
Special monitoring is essential for the patient with such type of problems
Use in Special Populations
Geriatric Use: No dosage adjustment is recommended in elderly patientless than 65 years of age.
Pediatric Use: Safety and efficacy have not been established in patients younger than 18 years.
Use in Patients with Impaired Renal Function: Obeticholic Acid has not been studied in patients with moderate and severe renal impairment (estimated glomerular filtration rate [eGFR] less than 60 ml/min/1.73 m2). In the population pharmacokinetic analysis, an eGFR greater than 50 ml/min/1.73 m2 did not have a meaningful effect on the pharmacokinetics of Obeticholic Acid and its conjugated metabolites.
Use in Patients with Impaired Hepatic Function: Obeticholic Acid is metabolized in the liver. Plasma exposure to Obeticholic Acid and its active conjugates, increases significantly in patients with moderate to severe hepatic impairment (Child-Pugh Classes B and C).The recommended starting dosage of Obeticholic Acid for moderate (Child-Pugh Class B) and severe (Child-Pugh Class C) hepatic impairment is 5 mg once weekly. If an adequate reduction in ALP and/or total bilirubin has not been achieved after 3 months of Obeticholic Acid 5 mg once weekly, and the patient is tolerating the drug, increase the dosage of Obeticholic Acid to 5 mg twice weekly (at least three days apart) and subsequently to 10 mg twice weekly (at least three days apart) depending on response and tolerability.
Overdose Effects
In PBC patients who received Obeticholic Acid 25 mg once daily (2.5 times the highestrecommended dosage) or 50 mg once daily (5 times the highest recommended dosage), a dose-dependent increase in the incidence of liver-related adverse reactions, including elevations in liver biochemical tests, ascites, jaundice, portal hypertension, and primary biliary cholangitis flare, was reported. In the case of over dosage, patients should be carefully observed and supportive care administered, as appropriate.
Therapeutic Class
Farnesoid X Receptor Agonists
Storage Conditions
Store at 15°C to 30°C in a dry place protected from light. Keep out of reach of children
- Type Tablet
- Tag
- Morbi leo risus
- Porta ac consectetur ac
- Vestibulum at eros